PART VI OF VI
JUNE 20, 2005
(Note that we'll be back in three weeks instead of the usual two). 
please:
pontificate
educate
illustrate
commentate (oh yeah)
and/or submit
by emailing us at tscq@interchg.ubc.ca
| | | | IN THE NAME OF FAIR GAME...
By Julie Hathaway, images by Jen Philpot
Doping to enhance athletic performance happens at every level of the game; lately, we have heard about it in professional baseball, at the Olympics, at other international competitions and even at our local gyms. Performance pressure is ever increasing, especially considering the financial endorsements and sponsorships associated with success. As a consequence, some athletes continue their attempt to better themselves through extensive training, drive and motivation, while others look for a little help from organic chemistry. International and national sports organizations, in conjunction with laboratories around the world are trying very hard to “crack” down on drug abuse in sports. The following is a brief overview of the most popular sport enhancing drugs, how they act biologically to strengthen the body, when they should be tested for and finally, what scientific equipment and methodologies are used for drug testing.
Steroids
By far, the most popular sports performance-enhancing drugs are steroids. They are not only commonly used in competitive sports but also in recreational weight training. Many studies have shown that the use of anabolic-androgenic steroids (AAS) by weight training individuals increases muscle mass and size by 5-20% over those not using any drugs[1].The AAS’ are synthetic derivatives of human testosterone, which is produced in the testes cells. The anabolic activities of AAS’ are of particular interest to athletes as they are involved in the stimulation of protein synthesis and the inhibition of protein breakdown, which contribute to muscle growth. The increase in muscle mass under the influence of AAS’ is due to muscle hypertrophy and the formation of new muscle fibers. It has been reported that AAS’ cause the incorporation of satellite cells (“reserve” skeletal muscle cells) into pre-existing muscle fibers to generate a higher number of cells in the tissue resulting in a stronger muscle1. AAS’ have been modified from testosterone so that they may remain intact in the body for a longer period of time, enabling them to produce their effects. These modifications include the addition of a methyl group (CH3), which slows down the steroid’s degradation by the liver, and alterations of the ring structures of testosterone, which increase their activity[2]. The structure of testosterone and the AAS derivatives that are banned in international sport are illustrated in Figure 1.
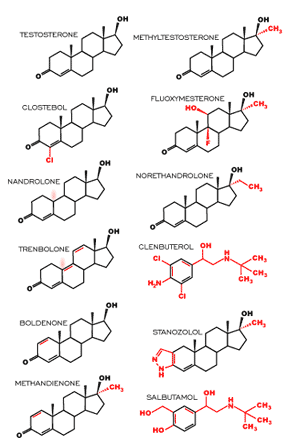
Figure 1. Testosterone and anabolic steroid derivatives
banned in international sport. Each derivative has been
slightly modified either by addition of substituent or through alteration of the ring structure (addition or removal of
double bonds, cleavage of bonds or addition of ring
structures)[3].
Detecting the presence of steroids is a difficult task. The analysis must be able to identify very small concentrations of drug as athletes might have discontinued use for quite some time prior to testing while still benefiting from the drug’s enhancing properties. Competition officials therefore need to test athletes for steroid use both during and before their competition times. The athlete’s urine is often the best sample to be taken for testing. Natural steroid levels (such as testosterone) for a particular population must be taken into account when testing for the presence of synthetic AAS. Often, the only way to determine “normal, natural levels” for the population of the athlete is through long term studies. The breakdown of steroids by the body is quite complex; often, only side products or the breakdown products (metabolites) can be detected. Therefore, the analysis must include carefully designed hydrolysis and extraction procedures which lead to the detection of desired, known metabolites of the AAS’ [3].
A commonly used method for detection of AAS metabolites is mass spectrometry coupled to gas chromatography.
Stimulants
Stimulants are another class of drugs that are often used by athletes for enhancing their performance. A lot of us consume a form of stimulant everyday when we enjoy a morning coffee or a Coke at lunch. Several of the banned stimulants are related to amphetamines. Amphetamines stimulate the central nervous system and mimic the sympathetic nervous system activities (the fight or flight responses). Amphetamines cause the release of dopamine in the brain and noradrenaline from the sympathetic nervous system, generating an overall feeling of arousal. This arousal is characterized by an increase in blood pressure, heart rate and metabolic rate. These drugs are believed to enhance performance through a reduction in feelings of fatigue[2].
Several studies have shown a 0.6-4% increase in performance in swimmers, runners and weight throwers given a source of amphetamine compared to those having taken a placebo[2]. In addition, the athletes reported an improvement in hand-eye coordination, strength and endurance.
To enhance performance, stimulants must be taken prior to an event as their effect is immediate. Therefore, testing for the use of stimulants must be done during the competition period. A urine sample is the best matrix in which to detect traces of the drug. Most of the above stimulants contain nitrogen and are quite basic in nature: these two features are exploited for detection testing. Analysis begins with an extraction into ether at a high pH, which results in an extract from the urine that should contain most of the volatile stimulants. Properties of the stimulants are quite different and thus require a method that allows for the detection of a wide range of compounds[3]. Traces of stimulants can be detected through mass spectrometry coupled to gas column chromatography, similar to the analysis of steroids mentioned above.
Diuretics
Diuretics are a class of banned sports enhancing drugs that are mainly taken by wrestlers or competitive weight lifters. Diuretics are capable of rapid water loss through the urine, resulting in a reduction in weight. This is particularly advantageous to the athletes in the aforementioned sports as it allows them to compete in a lower weight class. Diuretics are taken before the weight classification process and following this, the athlete re-hydrates himself to regain lost weight. Diuretics are capable of diluting urine by increasing renal flow. This makes banned drug detection much more difficult and may be used as a method of masking the presence of other drugs in the system. There are three major classes of diuretics. The first, the thiazides, block salt reabsorption in the distal tubules of the kidney, which promotes the excretion of sodium, chloride and potassium. The second are loop diuretics which block sodium chloride transport in the upper loop of Henle in the kidney, also promoting salt loss. Finally, the potassium sparing diuretics allows only sodium and chloride losses. Loss of salt is of course accompanied by a loss of water through osmosis[2].
Studies have shown that diuretics result in a body weight loss of 3-4% over 24 hours. Although the drugs appear to be doing the trick, this weight loss is accompanied by an increase in muscle temperature during exercise as well as electrolyte imbalance[2].
Due to their mode of action, detection of diuretics must be done prior to competition dates, especially since their solubility and their ability to dilute down urine makes them hard to detect in trace amounts. Often, other compounds in the urine make it difficult to detect diuretic compounds. Therefore, two methods must be used. First, liquid chromatography is used. Second, for confirmation purposes, the methylation of the aminosulfonyl group (O=S=O) is followed by the previously mentioned mass spectrometry coupled to gas chromatography (MS-GC)[3].
Catching those Cheaters…
In most cases, urine samples are collected from athletes according to a strict international protocol for doping control. For security purposes, a split sample is sent to the lab (sample A and sample B). Sample A is analyzed first. Sample B is kept frozen until sample A yields a positive result for a banned substance. At this point, the athlete is contacted and he or she has the right to request that sample B be opened and tested in front of witnesses[3].
The commonly used method of gas chromatography coupled to mass spectrometry for banned substance identification is also used in courtrooms to reveal evidence in cases of drug abuse, fire and explosives investigations and environmental analysis. The method has been around for many years and continues to be improved with the advent of technology.
Gas chromatography (GC) and mass spectrometry (MS) each perform very different tasks but act together to identify a particular substance.
The sample enters the GC component first through the injector port where it will be vaporized. An inert gas, (often Helium) then enters the column and sweeps the sample out of the injector and into the column. The carrier gas helps remove any water and impurities from the sample. The column allows for the separation of chemical compounds in the sample based on their affinity for the material inside the column. Compounds with higher affinity for the column material will exit slower than those with less affinity. In addition, the column is also gradually heated to allow another separation factor; compounds with lower boiling points will exit the column prior to compounds with higher boiling points. The rate at which the different sample components exit the system allows them to be measured and characterized. A gas chromatograph is pictured in figure 2. The components are then moved on to the MS[4].
In the first part of the MS, the ionizer, an electron beam causes the components to gain a positive charge. This particular process causes further breakdown of the individual components. Each component has a unique fragmentation pattern. The sub-components then enter a magnetic region where they are focused and sent to a detector. Components with a lighter atomic mass enter the magnet set up and will be sent to the detector first. At the detector, the component transfers its charge which then activates a recorder capable of registering atomic mass, based on the mass:charge ratio, and identify the concentration of the particular component in the sample. Steroids, stimulants and diuretics are all substances that can be detected by this gas chromatography-mass spectrometry method of analysis. A spectrum of atomic masses and concentrations is generated by the mass spectrometer that is carefully observed by the analysts. In most drug detection, a selective ion monitoring (SIM) analysis will be performed where only certain peaks are observed to see if they are similar to those obtained during the analysis of a banned substance[5].
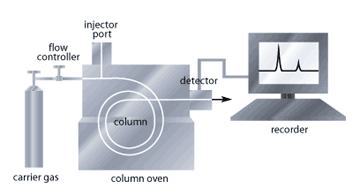
Figure 2. A schematic diagram of a gas chromatograph.
The drugs mentioned above are only three of many types performance enhancing drugs and performance enhancing methods that are currently banned for use in competitive sports. While new performance drugs and new harder-to-detect derivatives are sure to emerge in the next few years, it is hoped that technology can follow suit and improve its current detection methods. The media continues to report cases of sport doping, indicating that the problem still exists and further interventions and penalties must be enforced. It is the hope of many athletes and sport organizations around the world that soon, all cheaters will be caught and there will only be fair games to be played.
References
1. Hartgens F, Kuipers H. 2004. Effects of Androgenic-Anabolic Steroids in Athletes. Sports Medicine, 34(8), p. 513-554.
2. Clarkson PM, Thompson HS. 1997. Drugs and Sport: Research Findings and Limitations. Sports Medicine, 24(6), p. 366-384.
3. Trout GJ, Kazlauskas R. 2004. Sports drug testing-an analysts perspective. Chemical Society Reviews, 33, p.1-13.
4. Gas Chromatography, Sheffield Hallam University, School of Science and Mathematics, 1998, (accessed October 18, 2004)
5. Gas Chromatography mass spectrometry, All Science Fair Projects.com, 2004, (accessed October 18th 2004)
Julie Hathaway recently graduated with her B.Sc at UBC, and is probably much more exciting than this bio. Sadly, that is our fault as we asked for it much too late. | | | | For those that prefer a print version, please download our beautiful pdf file.
(part i pdf)
(part ii pdf)
(part iii pdf)
(part iv pdf)
(part v pdf)
home (again)
about (us)
archive (of stuff)
submissions (or suggest)
notes (on masthead)
bioteach (.ubc.ca)

|