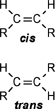| PART III OF VI
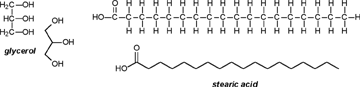
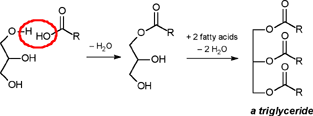
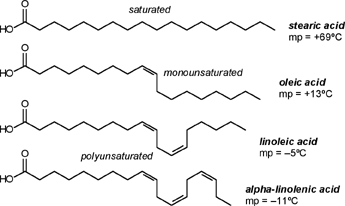
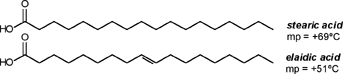
| A CHEMIST RESPONDS TO "A SCIENTIFIC EXPERIMENT." by W. Stephen McNeil (A review and/or rebuttal to “A Scientific Experiment” by Jaime J. Weinman, Issue One, Part II) Well, it's a good thing that science experiments on webpages aren't subject to the traditional anonymous peer review process. It they were, Jaime would probably get a reply something like this: "Dear Mr. Weinman, "We regret that we cannot accept your manuscript for publication in its current, or likely any, form. The reviewers have pointed out a number of glaring deficiencies and omissions, briefly summarized below. "Although your scientific curiosity is to be applauded, your experimental methodology seems exceedingly limited, and lacks many important details. Your report describes only a single experiment, with no repetition or statistical analysis, and no meaningful description of the nature of the sample (beef or pork? smoked or unsmoked? jumbo or regular?). Similarly, your description and discussion of results is purely qualitative and exceedingly terse. What mass of fluid was extracted from the hot dog? What was the initial mass and volume of the sample? The fluid is characterized solely as "fat", but no analytical details are provided to support this conclusion. Your characterization of the final product as "literally, a stick" was met with particular disbelief from one reviewer, who points out that a microwave-induced transformation of protein to cellulose would defy all known laws of chemical and biochemical reaction. Finally, your conclusion seems to be little more than "hot dogs have a lot of fat in them", a conclusion of little or no scientific merit in that it would be immediately apparent to any numbskull who reads a nutrition label. "In conclusion, we feel that your experimental design and implementation would rank well below par at any high school science fair, and recommend that for your next project, you try building a trebuchet. Because trebuchets are freaking cool." But that's mostly because the anonymous peer reviewers for scientific manuscripts can sometimes be real pricks. Truth be told, there are actually some cool things to be learned from this. I'm not sure "don't eat hotdogs because they're, like, all fatty and gross and stuff" is one of them (because you should already have known that) but it's a nice experimental confirmation of that nutritional info, which, to be fair, is something that nobody ever bothers to read and doesn't actually tell you what you should know, unless you've taken a fair amount of biochemistry. In brief, meat is pretty much just protein, fats/oils (aka lipids), and water. The microwave heats up the water, which heats up the rest of it, which once the temperature gets hot enough will then melt all the fatty material. (How does the microwave work? You should ask How Stuff Works, of course.) This, of course, is when the now liquid mixture of fats, oils, and water starts oozing out. Repeated enough times, this will extract all the fluid, and the remainder will be overcooked zero-moisture hardened protein. - with, you know, some onion power and stuff. Which would actually happen with any kind of meat. Zapping anything in the microwave for long enough makes it unappetizing. Starting with something already unappetizing doesn't help. Besides, if you were in the mood to watch oozing fat, it seriously doesn't get much better than hotdogs. A random hot dog package I looked at yesterday more or less broke it down as follows: A typical hot dog is about 35 to 40g, and supplies your body with about 100 calories of energy. (Except that they aren't really calories; they're kilocalories. That is, every nutritional "calorie" is actually 1000 calories, equivalent to the amount of energy it takes to increase the temperature of one litre of water by one degree Celsius. Why or how this stupidity arose has never been explained to me.) Of that mass, 8.5g is fat, or about 20-25% by mass. However, fat supplies your body with about 9 calories per gram (more than twice as much as a gram of protein), which means that the fat represents something like 75% of the total calories. "A stick injected with fat" has it backwards. More like "fat wrapped in sausage casing for your dining convenience". Of that total fat, there are about 3.7g of saturated fats (the really bad stuff), about the same of monounsaturated (much of which may be trans fats, which are almost as bad as the fully saturated and will most likely soon be listed separately on the label to help you avoid them). Notably, hardly any of it is the (relatively) healthy oils, the polyunsaturated fats. Which is just about the point at which your eyes start to glaze over and you quit trying to sort out what the nutrition label tells you, and instead you get distracted by the next package of hotdogs, because those ones have !!!omigod cheese right inside the wiener!!! and you buy those instead. Which is a shame, because there's some cool and pretty easy-to-grasp chemistry on that label. Fats and oils are triglycerides. They are compounds that arise from a reaction of glycerol with three molecules called fatty acids. Fatty acids are long chains of carbon atoms, most of which are connected to hydrogen atoms, except for one at the end of the molecule, which forms a carboxylic acid group, COOH. Below are two depictions of both glycerol and a fatty acid called stearic acid, which has eighteen carbon atoms. In the first drawings, all the C and H atoms are shown explicitly, but writing them all out like that is a giant pain, so nobody does it. In the second drawings, a standard shorthand is used, where lines represent bonds between carbon atoms at the vertices, and the Hs connected to those C atoms are omitted. They're still there, and we can infer their presence because each carbon atom forms four bonds -- if you don't see four bonds explicitly drawn, the missing ones are connecting to H atoms.
| For those that prefer a print version, please download our beautiful pdf file. (part i pdf) (part ii pdf) home (again) about (us) archive (of stuff) submissions (or suggest) notes (on masthead) bioteach (.ubc.ca)  A SUBMISSION EXPERIMENT PHYSICS ENVY AMONG BIOLOGISTS: FACT OR FICTION? by T.J. Nelson EUPHEMISMS THAT ALSO SOUND LIKE STRANGE TISSUE ENGINEERING PROJECTS by David Ng IN WHICH OUR PROTAGONIST LEARNS THE IMPORTANCE OF THE BASE CASE. by Moebius Stripper MYSTERY ORGANISM BAFFLES GIRL ADVENTURER. by Bethany Lindsay HIPPOPOTAMUS by Carolyn Beckman |