THE BUSINESS OF BIOTECHNOLOGY
(August 2004)
Beer was created in 2000 B.C. when Egyptians first discovered how to ferment yeast. In 500 B.C., the Chinese invented a crude antibiotic from moldy soybeans curds and used it treat boils. These discoveries indicate an early human awareness of biology’s usefulness in creating medical and agricultural advances and for over four thousand years the industry of biotechnology has flourished, growing into a multibillion-dollar cutting edge industry over the last 30 years.
The biotechnology revolution was heralded by two cornerstone discoveries: recombinant DNA and hybridomas1. Lucrative product applications had an immediate impact on industrial and agricultural developments, but were most significant in the health care industry. While the possibilities of the biotech revolution are numerous, its products must abide by restrictions imposed by government agencies on their development2. New drugs must pass stringent clinical trials before they can be sold to the public. Companies developing these drugs must also negotiate the vagaries of market forces while leading scientific research into promising areas.
The Roots of the Biz
The first generation of biotech companies were created in the 1970’s in response to the cornerstone breakthroughs that occurred in 1973. Stanley Cohen and Herbert Boyer started the recombinant DNA ball rolling by perfecting techniques involving restriction enzymes and DNA ligases that allowed them to cut and splice together segments of DNA. This innovation was swiftly followed in 1975 by George Kohler and Cesar Milstein’s successful fusion of a single antibody-producing cell with a myeloma cell (a type of white blood cell) [1]. The resulting hybrid cell or hybridoma took the form of an immortal cell line capable of producing antibodies for specific targets, often referred to as monoclonal antibodies.
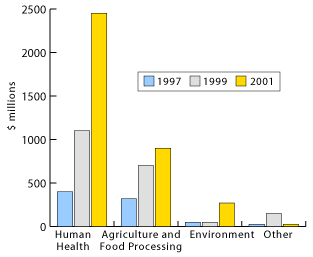
Both recombinant DNA and hybridomas formed the basis for producing highly sought after human proteins such as human growth hormone and insulin [1]. From a business perspective, advancements in genetic engineering made it commercially feasible and highly lucrative for biotech companies like Genentech, Amgen and Genzyme to develop proteins as pharmaceuticals. A whole new world of unexplored research and vast new markets for pharmaceutical products was created. From the early stages of first generation biotech companies, the growth of the industry has continued at a booming rate (see Table 1).

New Directions in the Biotech Industry
The growth of the biotech industry in combination with genetic engineering research promises to deliver new and innovative health care products. Gene expression using biochips and microarrays, antisense drugs, gene therapy, signal transduction, and combinatorial chemistry are just a few examples of promising and cutting-edge biotech research [3]. But before the public will see the result of these endeavors, such as a new drug, biotechnology products must go through stringent government approval, passing through a standard development process [4].
The Drug Development Process
The type of biotech product developed dictates which legal requirements must be met. For example, the Environmental Protection Agency (EPA) must approve agricultural products, whereas, a new drug must pass the Food and Drug Act (FDA)—administered by Health Canada—and associated clinical trials before reaching the general public [4-5]. There are four stages to the drug development process:
1. Discovery and Early Research;
2. Pre-Clinical Testing;
3. Phases I, II and III of Clinical Trials; and
4. Phase IV – Approval & Post-Marketing Testing.
The timeframe of moving through this process is extensive, taking years to complete (see Figure 2).

Discovery and Early Research Stage
The whole process begins with basic research, in which the causes of diseases are targeted, studied, and then drug therapies envisioned and created. Intellectual property issues must be addressed at this stage to prevent ideas from being appropriated by someone else [6]. There are four ways to protect a discovery: (i) patents, (ii) copyrights, (iii) trademarks, and (iv) industrial design (ref). Patents are most commonly used for biotechnology and can protect an innovation for twenty years.
Preclinical Testing
Once an idea has been developed, it must move from the test tube to animals. The purpose of preclinical testing is to prove to Health Canada that it is safe on animals and to gain insight into the workings of the drug as it is metabolized [4]. A well-developed preclinical study will generate information on what doses of the drug are safe and which create toxic side effects. Researchers must pay attention to how the drug is broken down by the animal and determine if toxic metabolites are created. The time within the blood system and loss of effectiveness depending upon the modes of drug administration (oral-digestion, intravenous vs. subcutaneous or inhalation) are also observed. From this preclinical testing stage, Health Canada will require data on the stability of the drug compound and other physical properties including its market potential [4]. If the drug does not provide significant benefits over existing drugs, it may be rejected before clinical trials to conserve valuable R&D funds.
Clinical Trials – Phase I
Once the FDA or Health Canada approves a drug for clinical trials, a small group of 20-80 generally healthy volunteers is used to check the safety of the drug and determine any serious short-term side effects [5,7]. The clinical trials must also address:
– Whether the drug works well enough to be given to patients.
– Whether the potential risk of toxic side effects outweighs the therapeutic benefit.
– Which dose regimen and dose administration provides the best response and the fewest side effects.
– Whether the drug is better, equivalent or worse than existing treatments.
An additional analysis imposed by health-care insurers called the pharmaeconomic analysis is conducted to determine whether the additional cost of the drug will be balanced by the lowered total cost of care. The overall purpose of phase I clinical trials is to learn the best way to use the drug and to determine whether the drug is safe on humans.
Clinical Trials – Phase II
If a drug passes phase I testing and is deemed safe for human consumption, the drug is then tested for how it behaves on patients in phase II clinical trials [5,7]. Phase II studies involve 100 to 500 patients that are divided into subgroups and given the drug in different doses, in different ways, and on different schedules. Such research is normally done using double-blinded studies, where both the patients and doctors have no knowledge about the treated and control (placebo) groups.
The data obtained in phase II clinical trials is gathered for the primary purpose of designing optimal phase III clinical trials, with the studies demonstrating how people in the “real world” will utilize the drug. While companies may report a hint of the promising nature of a drug under testing in phase II to improve its market confidence, it is important to note that the data gathered does not provide definitive proof of the drug’s efficacy.
Clinical Trials – Phase III
The purpose of phase III clinical trials is to gather statistically significant proof that the drug is effective enough to warrant its use and is safe enough for public consumption [4-5]. This stage is known as the “pivotal trials” because the data generated from this stage is used to obtain approval under the FDA to market the drug.
The drug division of the FDA, responsible for small-molecule drugs, typically requires two Phase III trials before considering a New Drug Application (NDA) [4]. Where no existing treatment exists for life-threatening diseases, exceptions to the requirements are made at this stage, but careful evaluation and post-marketing studies (Phase IV) must be conducted as a condition of the approval after Phase III.
Health Canada will evaluate whether the testing methods were consistent to eliminate bias and provide a good comparison with the control group [4]. Because in some cases it would be unethical to have a control group that is given a placebo or no treatment at all, it is common that the drug has to be compared with existing treatments. The benefits can range from greater effectiveness on the disease or symptoms to greater effectiveness because of the method of administration or other appealing physical properties. Finally, a pharmaeconomic analysis must prove that the drug is cost-effective in disease treatment.
Approval & Market – Phase IV
If a drug passes phase III clinical studies and the NDA is approved, the drug can be marketed for sale to the public [4-5]. Most drugs are conditionally approved and must be continually monitored for unanticipated problems, with long-term affects and activity in the larger population being studied. In fact, even after vigorous stages of testing and stringent regulatory scrutiny, some drugs are used in non-prescribed ways that may cause unanticipated problems. For example, Viagra was a drug designed for men, and was never tested on women. When women ingested the drug, certain side effects did occur. Approvals for the drugs Redux and Pondimin have been withdrawn because their combination with other weight loss drugs caused heart valve defects, a side effect that could not have been predicted during the clinical trials.
Summary of the Drug Development Process
The average length of time from discovery to market is twelve years, with a financing of $500 Million dollars8. This is an arduous process that places safety first and thus does take time. Table 2 below summarizes the key stages:

Drug development comprises a significant share of the biotech market and investment. The product development process is long and risky as many products fail clinical testing, even at the final stages. In addition to passing scrutiny on the drug’s efficacy, the drug must also compete with existing treatments, or in the case of no treatments, must be closely monitored even after approval.
A Long Road…
Biotechnology companies begin with an idea or a discovery, which they protect by patents. A product may take twelve year to produce and with the eventual end of the patent protection at 20 years, a biotech company must have discoveries and eventual product always on the go. This continual need for new products in the pipeline is necessary for its survival and requires creative financing and excellent long-term business management. In this respect, the biotech industry faces unique challenges. Not only must it market and sell its products, it must also invest heavily in ideas that take years to show potential. Nevertheless, from its historical development in agriculture and medicines, to its innovative revolution in the 1970’s, the biotech industry continues to offer promising scientific solutions for industrial, agriculture and health care issues. In answer to the question of how this is done, we find the answer is complex, expensive and extremely exciting.
Additional Reading
1. Turner L.1999. Building a better biotech company. Nat Biotechnol 17 Suppl: BE5-6.
2. Rosendal GK. The politics of patent legislation in biotechnology: an international review. Biotechnol Annu Rev 1: 453-76.
3. Rozovsky FA, Rodney K. 2003. Clinical trials and human research: a practical guide to regulatory compliance. San Francisco: Jossey-Bass.
4. Fecenko MJ. 2002. Biotechnology law: corporate-commercial practice. Markham, Ont: LexisNexis Butterworths. 316p.
References
1. Bud R. 1993. The uses of life: a history of biotechnology. New York: Cambridge University Press. 299p.
2. Murray T, Mehlman MJ. 2000. Encyclopedia of ethical, legal, and policy issues in biotechnology. New York: John Wiley & Sons.
3. Borém A, et al. 2003. Understanding biotechnology. Upper Saddle, NJ: Prentice Hall. 216 p.
4. Descriptions of various Health Canada Acts and Regulations.
5. Friedman LM, et al. 1998. Fundamentals of clinical trials. New York: Springer. 361p.
6. Brown WM. 2003. Intellectual property law: a primer for scientists. Mol Biotechnol 23(3): 213-24.
7. Good PI. 2002. A manager’s guide to the design and conduct of clinical trials. Hoboken, NJ: Wiley-Liss. 228p.
8. Grossmann M. 2003. Entrepreneurship in biotechnology: managing for growth from start-up to initial public offering. Heidelberg/New York: Physica-Verlag.
(Art by Jiang Long)