PREIMPLANTATION GENETIC DIAGNOSIS AND OUR FUTURE: SHOULD WE BE PEERING INTO THE WOMB?
(August 2003)
“Those who cannot remember the past are condemned to repeat it.”
— George Santayana
“In the realm of bioethics, the evils we face are intertwined with the goods we so keenly seek. Distinguishing good and bad thus intermixed is often extremely difficult.”
— Leon R. Kass MD, PhD
Chairman, The President’s Council on Bioethics
Since the announcement that the entire human DNA genome had been sequenced in June 2000, newspapers around the world have been rife with proclamations describing how this information is being used for the prevention and treatment of genetic disorders. Among the most promising and controversial claims is the ability of genetic technology to screen in vitro conceived embryos for the presence or absence of certain genes before implantation into the mother. However, accompanying these new technologies are a number of very old questions.
Lessons from the Past
Eugenics is not a new phenomenon. It is the term used to indicate the genetic improvment of the human race by controlled selective breeding [1]. Believers in eugenics seek to apply the techniques commonly used in animal husbandry and plant development to human beings. The eugenic movement was very popular throughout North America during the late nineteenth and early twentieth centuries [2]. The popularity of the eugenic movement can be attributed to a number of social and technological developments that coincided with the spread of the industrial revolution throughout the United States from approximately 1865 to 1890.
Science and technology made breathtaking strides in the latter half of the nineteenth century. So confident were people in the abilities of science to solve the problems of the world that in 1899 Charles Duell, head of the US patent office, suggested that his office be abolished, saying, “everything that can be discovered, has been discovered.” Clearly, Duell’s recommendation was arrogant and premature, but his statement reflects an attitude shared by many of his time. Life expectancies were rising thanks to the development of better public health practices. Incomes throughout the United States were rising due to the increased production afforded by industries at the time. Electricity was being made available throughout the country, increasing the overall quality of life for the newly affluent country.
However, in stride with these advances a number of disturbing developments were growing. Social upheavals throughout Europe led to increased unemployment among the working classes. Immigrants from Europe, particularly Eastern Europe, came to the U.S. seeking a better life. Although the U.S. needed immigrants and the cheap supply of unskilled labour they provided, the country was not prepared for the approximate one million people per year who were entering. Moreover, the jobs held by the immigrants were often the most dangerous, with high risk of illness or death. The rapid growth rate also led to many problems that persist during times of upheaval, such as alcoholism, prostitution, and an elevated crime rate. Darwin never really embraced eugenics as a way of dealing with societal problems, but others, observing problems associated with the rapid influx of immigrants, began to manipulate Darwin’s evolutionary theories as a way of rationalizing a controversial eugenic management method.
With the increased crime and poverty levels existing primarily amongst the recently arrived immigrants it was thought by many at the time that these problems existed because of a defect in these people [1]. According to them, it was society that ultimately paid the price for those who came to be known as “defectives” or “degenerates,” society deserved a solution. The irony was clearly lost on those at the time who looked to improve society but chose not to examine society itself. Thus, policies were developed that sought to limit the effect of inferior genes on American (read “white”) society [1]. These included immigration restrictions from certain countries, forced sterilization of inmates, marriage prohibitions, and racial segregation, the effects of which are still currently being felt.
Are Genetic Advances Leading Us Down the Same Road Again?
Although the science of eugenics was questionable in its interpretation of social reform and the mechanisms of heredity, it is important to note that at the time eugenic policies were developed, nothing was known about the nature of how traits were transferred from parent to offspring. Thus, one must consider the ignorance of the investigator who originally drew conclusions about controlling heritable traits in society.
Recent developments in reproductive genetic technology have raised concerns among some circles that the eugenic movement may be entering a renaissance [1]. However, this time around, rather than poorly defined diseases like “feeblemindedness,” we have clearly defined diseases with a known genetic component that can be identified, and in some cases treated. Orators of the new eugenics suggest another possibility, apart from identification and treatment, whereby diseases with a strong inherited genetic component can be eliminated entirely by preventing that gene from ever being passed on again.
Pre-implantation genetic diagnosis (PGD) is an extension of previously existing prenatal screening technologies. Prenatal screening in an uncomplicated pregnancy usually involves ultrasound to examine fetal development and assess whether there is any abnormal development. In cases where these is a likelihood of abnormal development, as in women over the age of 35 or those with Down’s syndrome or spina bifida, chorionic villus sampling or amniocentesis may be performed. These techniques involve the removal of a sample of chorionic villus or amniotic fluid from a pregnant woman. A sample of which contain cells that have come from the developing embryo, which can be analyzed for the presence of abnormalities [5].
During the 1950’s and 1960’s, these techniques could only be used to determine the sex of the fetus or whether the cells contained sizeable chromosomal defects [5]. Thus, individuals at risk of transmitting X-linked diseases like Hemophilia could determine whether or not they carried a male child, or those at risk of pregnancies with a gross chromosomal defect like Down’s syndrome could examine the fetus for these types of genetic abnormalities. The difficulty of obtaining abortions at this time made the ability to look for these types of problems primarily a tool for preparing expecting parents for the likelihood of having a child with a chronic congenital disability.
With advances in molecular biology in the 1970’s and early 1980’s, such as the development of polymerase chain reaction (PCR) and DNA sequencing, a much larger number of diseases were described at the genetic level. Soon to follow were tests for the presence of these diseases-associated genes, making prenatal screening a much more powerful tool [5]. These technological advances also coincided with the 1973 Supreme Court of the United States decision legalizing abortion on demand in that country.
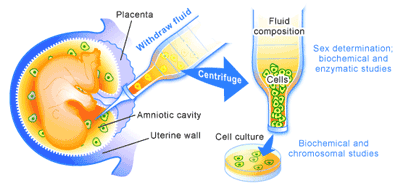
In putting these two developments together, a moral conundrum was created. The combination made it possible to determine whether or not a child was healthy before it was born. Therefore, if a problem was found, that pregnancy could be terminated. For those individuals opposed to abortion, this was a disheartening development, since, in addition to the various social and economic reasons used to justify abortion, a new health-related reason was being created. Groups advocating the rights of people with disabilities foresaw the advancement of a Gattaca-like society, in which individuals without a desired genetic complement would be relegated to underclass status.
Gene Tests and Medical Science Today
Diseases that can be screened for today include cystic fibrosis, Tay-Sachs disease, Huntington’s disease, and a host of other devastating ailments [5]. Using PGD to screen against these diseases is relatively uncontroversial in the United States, because there is no regulation of fertility treatment. In the UK, the situation is somewhat different. A government body, the Human Fertilization and Embryology Authority (HFEA), was established in 1991 and monitors all labs providing fertility treatments and PGD [6]. It also licenses and monitors all embryo research.
In the UK, it is legal to screen in vitro fertilized (IVF) embryos for the presence of a disease gene and to implant only embryos that are healthy. However, improvements in screening have opened up a number of other applications for PGD. Take Thalassemia for instance. It is a genetic disorder that results in underproduction of the beta subunit of hemoglobin, resulting in a much lower ability to carry oxygen throughout the body. Treatment involves bimonthly blood transfusions, which unfortunately carry a side effect in the form of iron overload. This is treated by chelation therapy, but the disease is often fatal.
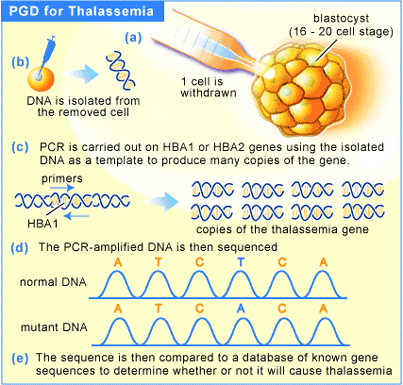
Treatment for the disease used to consist of bone marrow transplants, a treatment that is not very successful because the patient rarely survive the initial radiation treatment that allows the transplant to take place. More recently, umbilical cord blood has been shown to be a viable treatment for thalassemia [12]. By using PGD to screen embryos that are a perfect blood match for an affected individual (usually a sibling), the chances of finding a perfect match are greatly increased.
In the US, PGD was successfully used to screen embryos for both the absence of the Thalessemia disease gene and for a perfect tissue match for an older, diseased sibling [7]. The result was a healthy baby, with the umbilical cord blood transfusion giving his older sibling a 90% chance of survival. This marked the first case whereby a PGD was used not only to screen for the possibility of disease, but also for what could be termed a non-health related screening. Are we poised at the edge of a slippery slope? It is not a great leap to imagine that we are, and that screening for other “desirable” traits is close at hand. Since there is little regulation in private fertility clinics in the United States, it will require a great deal of political will to address this problem at this level.
Are we Already Sliding? The Case of the Hasmi Children
Indeed, the complexity of the issue is best illustrated by a recent series of court cases in Great Britain [8]. A couple from Leeds, Raj and Shahana Hasmi who have a son with thalassemia wanted another child. They realized that they were both carriers of the disease and wanted to ensure that their next child was free of the diseased thalassemia gene. To do this they wanted to use genetic technology to allow them to find an embryo that was free of the disease and that would be able to serve as a blood donor for their son. In this way, they hoped to have a healthy child and also harvest umbilical stem cells to cure their first son of his disease. If it worked in the US, they saw no reason why it couldn’t work in Britain.
Alarm bells started ringing at HFEA when the proposal for the technique was submitted for approval. The request was put on hold to allow the development of a policy toward the new technology. In December of 2001 policy was decided and the HFEA allowed the procedure to go ahead. However, one year later, following lobbying from the Comment on Reproductive Ethics (CORE), the British high court stepped in and ruled that the HFEA did not have the authority to allow PGD for the purposes of creating a perfectly matched donor [11]. The Hasmi’s were prevented from using the treatment. Not to be deterred, the Hasmi’s took their case to the Court of Appeal and again gained a ruling in their favour. In April of 2003, the HFEA, cautious after their previous decision had been overturned by the high court, ruled out widespread use of the technology but permitted the Hasmi’s to use the technology [10].
The period of time between the Hasmi’s application coming forward and the development of the current policy toward IVF and PGD, over 3 years, demonstrates the gap between the pace of technological advancements in genetics and the policies that will regulate it. Often the complexities of the science itself can mask the ethical questions that are raised by its application. In the case of the Hasmi’s, many questions are raised apart from the controversy associated with the use of the technology itself. What is the status of the embryo created? Is it a person with its own rights, or is it created merely as a means to an end? With the Hasmi’s intention to use umbilical cord blood there is no risk to the child that would result from the pregnancy. However, it is not difficult to envision a case in which embryonic stem cells would be the desired product. What would be permissible in this case? What if a kidney were required? A kidney transplant would entail some risk to the donor child, but failure to provide a perfectly matched kidney could result in the death of the older sibling. Even the fitness of the parents to make decisions on behalf of both of their children may be called into question. Many people foresee a future in which there will be two classes of children, the privileged who get to live, and others who are destined to be used for spare parts.
It is clear from this one case that there will be no easy answers to the questions that these developments have raised. In the UK, the back and forth battles among different regulatory agencies and judicial levels indicate that there will continue to be new developments in how these technologies will be used. It is heartening however, to note that the success of the HFEA in contributing to the development of strong policies on the use of PGD has prompted the development of similar agencies in other countries. While there is still no official agency dealing with these technologies in the United States, in November 2001 President Bush established the President’s Council on Bioethics [9]. Its first recommendations in July 2002 reflected the deep divisions that exist in the United States with respect to reproductive technology. In Canada, Health Canada has begun consultations to determine the structure of a regulatory agency to govern these technologies, but no unified policy has been released to date [10,11].
Society is a reflection of each member that it contains. It is very important when determining the applications of new technologies, to conduct debates in an open and honest way. Reproductive technologies present a number of very important and exciting opportunities for treating and preventing disease, but that excitement must be tempered by a full and frank discussion of what the consequences of their use may be. Just as those who once espoused eugenic principles could not foresee the negative outcomes of those policies, we must be wary of the possibility that decision we make now could seriously harm our society’s future. We must remain vigilant and engaged to ensure that this does not happen.
Additional Reading
1. Gould S.J. (1996). The Mismeasure of Man. New York: W. W. Norton Publishers. 444p.
2. Lynn R. (2001). Eugenics: A reassessment. Westport, CT: Praeger Publishers. 366p.
References
1. Lynn R. (2001). Eugenics: A reassessment. Westport, CT: Praeger Publishers. 366p.
2. Lombardo P. Eugenics Laws Restricting Immigration. Image Archive of the American Eugenics Movement.
3. Darwin C. (1859). The Origin of Species. London: J. Murray [1901]. 703p.
4. Darwin C. (1882). The Descent of Man. Amherst, NY: Prometheus Books [1998]. 698p.
5. The Genetic Drift Newsletter. 2001. Prental Diagnosis Vol.19 (Spring):
6. Human Fertilization & Embryology Authority
7. Belkin L. The Made to Order Saviour. New York Times Magazine. July 1, 2001.
8. BBC News. Family vow to have designer baby. Jan.10, 2003:
9. The President’s Council on Bioethics
10. Health Canada. (1999). Reproductive and Genetic Technologies Overview Paper
11. Health Canada, 2002. Press Release.
12. Goussetis E, Peristeri J, Kitra V, Kattamis A, Petropoulos D, Papassotiriou I, Graphakos S. (2000). Combined umbilical cord blood and bone marrow transplantation in the treatment of beta-thalassemia major. Pediatric Hematology & Oncology 17(4): 307-14.
(Art by Jane Wang – note that high res versions of image files available here)